- Home
- News
- Spotlight on Science
- X-ray spectroscopy...
X-ray spectroscopy studies ruthenium catalysts during nitric acid production
19-02-2024
Scientists have used the non-invasive technique of in-situ X-ray absorption spectroscopy at beamline BM31 to investigate the redox transformations of ruthenium catalysts during the oxidation of nitric oxide to nitrogen dioxide, a key step in the production of nitrate fertilisers for agriculture. A deeper understanding of the process could lead to higher-yield, more efficient fertiliser production.
One of the main objectives in the drive towards more sustainable chemical processes is to intensify the processes themselves, thereby reducing their carbon footprint and increasing energy efficiency. One such example is the oxidation of nitric oxide, one of the main steps in the chemical process that produces industrial nitric acid [1,2]. Nitric acid is a corrosive mineral acid mainly used to produce nitrate fertilisers, which dramatically improve agricultural output in modern agrarian systems.
Commercial nitric acid production uses the century-old Ostwald process, which consists of three important chemical steps: catalytic oxidation of ammonia using a Pt-Rh gauze catalyst; followed by gas-phase oxidation of NO to NO2 using a series of heat exchangers, and finally, NO2 absorption in water to produce nitric acid.
Catalysing the bulky homogeneous gas-phase oxidation of NO to NO2 may lead to about a 15% intensification of the Ostwald process [1]. Supported Ru catalysts show promising activity and stability at ambient and 4 bar(g) pressures at industrial nitric acid production conditions.
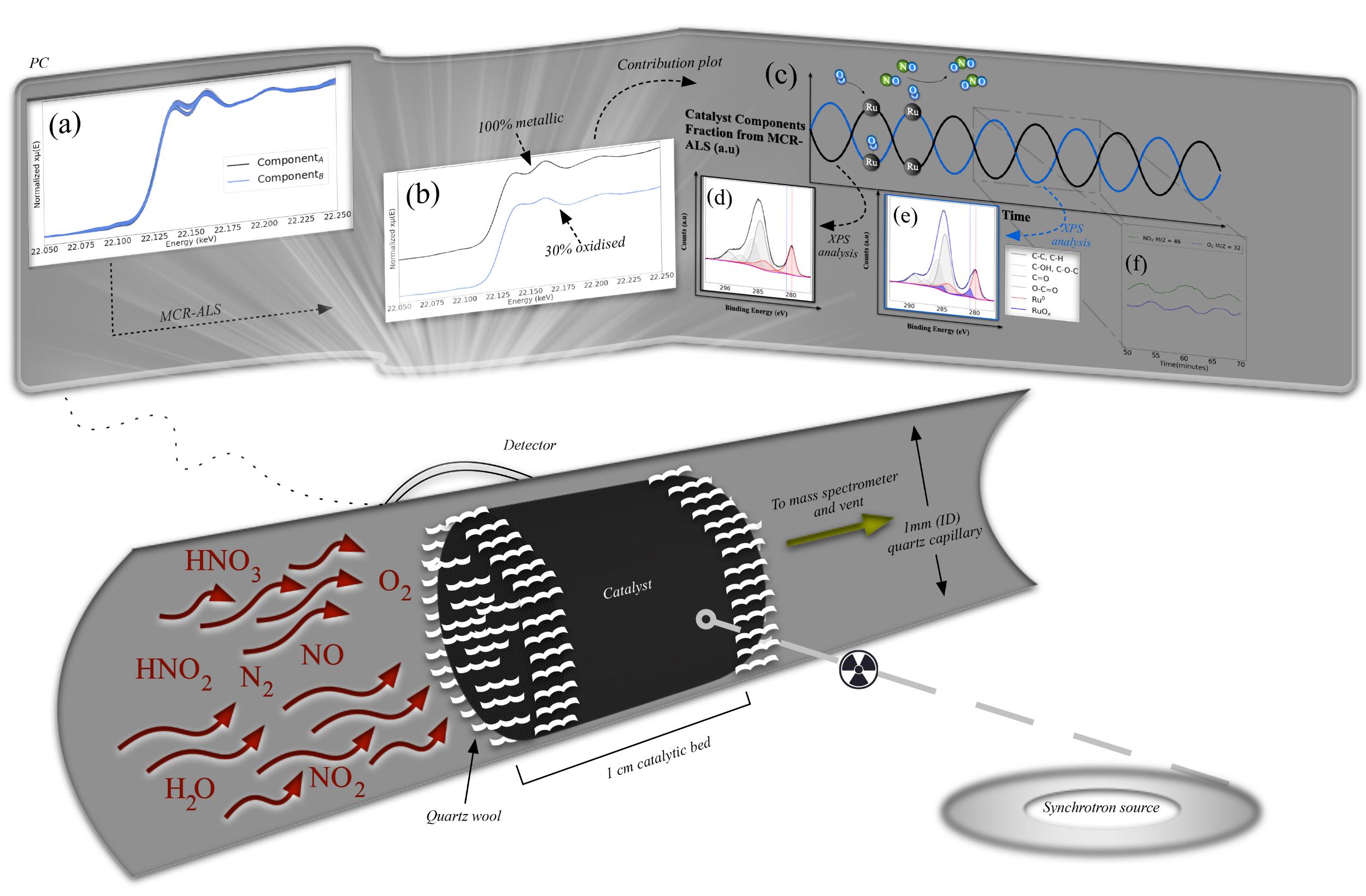
Experiments at the ESRF have helped to decipher the enigma behind the Ru catalyst’s capacity to oxidise nitric oxide at industrially relevant conditions, thus paving the way to intensifying a large established industrial process [1,2]. In-situ X-ray absorption spectroscopy (XAS) at the Ru K edge (22.1172 keV) was carried out at the Swiss-Norwegian beamline (SNBL) BM31.
Figure 1 illustrates the experimental setup: X-rays continuously irradiated the 𝛾-Al2O3-supported Ru catalyst during NO oxidation. An X-ray absorption near-edge structure (XANES) spectrum was collected every 8-10 seconds, precisely recording the changes in the Ru in the catalyst sample at isothermal conditions. A mass spectrometer (MS) was used to analyse O2 and NO2 in the product process gas.
Click figure to enlarge
Fig. 1: In-situ X-ray absorption experimental setup for oxidation of nitric oxide at beamline BM31.
Multivariate curve resolution-alternating least squares (MCR-ALS) data analysis of the in-situ XANES data collected during the experiment revealed two distinct components of Ru (as presented in Figure 2a). Component A was completely reduced (representing Ru0 in the metallic state) and component B was 30% oxidised. By synchronising the XANES and MS data acquisition rate, subtle changes could be observed in the Ru during NO oxidation. Figures 2c and 2f display the synchronised MCR-ALS contribution plot and the MS signals of O2 and NO2 in a 20-minute time frame. The oxidation state of a fraction of Ru in the catalyst oscillates between slightly oxidised and completely reduced. To understand the oscillating behaviour, extended X-ray absorption fine structure (EXAFS) spectroscopy and X-ray photo-electron spectroscopy (XPS) analyses of the two components were performed, and a clear surface oxidation was observed in component B (as presented in Figures 2d and 2e).
Click figure to enlarge
Fig. 2: a) In-situ XANES profiles of 𝛾-Al2O3-supported Ru catalysts collected during NO oxidation at 350°C. b) MCR-extracted components from the XANES data in (a). c) MCR-calculated contribution plot across 3 hours of XANES data collection. C 1s and Ru 3d XPS spectra of (d) component A and (e) component B. f) Collected mass spectrometer signal for O2 and NO2 during 20 minutes of a total of 3 hours NO oxidation.
The results reveal the mechanism behind NO oxidation at industrial nitric acid production conditions over 𝛾-Al2O3-supported Ru catalysts, and suggest a method to further tune the performance of the Ru catalysts at demanding reaction conditions. Furthermore, the study demonstrates that with careful experimental design and data analysis from complimentary techniques, a bulk technique such as X-ray absorption spectroscopy can also probe the surface of the sample. Overall, the work highlights the capacity of in-situ X-ray tools to bridge the gap between laboratory- and industrial-scale reactions.
Principal publication and authors
Redox transformations of Ru catalyst during NO oxidation at industrial nitric acid production conditions, J. Gopakumar (a), P.M. Benum (a), I.-H. Svenum, (b), B.C. Enger (c), D. Waller (d), M. Rønning (a), J. Chem. Eng. 475, 146406 (2023); https://doi.org/10.1016/j.cej.2023.146406
(a) Norwegian University of Science and Technology (NTNU), Department of Chemical Engineering, Trondheim (Norway)
(b) SINTEF Industry, Materials and Nanotechnology group, Trondheim (Norway)
(c) SINTEF Industry, Kinetic, and Catalysis group, Trondheim (Norway)
(d) YARA Technology Center, Porsgrunn (Norway)
References
[1] C. Grande et al., Ind. Eng. Chem. Res. 57, 10180-10186 (2018).
[2] J. Gopakumar et al., Catal. Sci. Tech. 13, 2783-2793 (2023).
| About the beamline: BM31 |
| This experiment was performed on the new BM31 end-station, installed in 2022. BM31 is specialised in combining X-ray absorption spectroscopy (XAS), high-resolution powder diffraction (HRPD), and total scattering. The optics and end-station can automatically switch between various configurations. This enables measurements of XAS, HRPD and total scattering datasets in a combined time-resolved manner on the same sample under the same experimental conditions. A set of sample environments is available for temperature-dependent, catalytic and electrochemistry experiments, as well as a gas distribution and analysis system. |